J Biomed 2019; 4:7-13. doi:10.7150/jbm.29161 This volume Cite
Review
Gene Therapy for Hepatocellular Carcinoma: An Update
1. 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
2. Department of Obstetrics and Gynecologic Surgery, “G. Papageorgiou” Hospital, Aristotle University of Thessaloniki (AUTh), Thessaloniki, Greece
3. 1st Internal Medicine Division, University Hospital of Ioannina, University of Ioaninna, Medical School
4. Surgery Department, “Interbalkan” European Medical Center, Thessaloniki, Greece
5. Pathology Department, Faculty of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
6. Pulmonary - Oncology Department, “Theageneio” Cancer Hospital, Thessaloniki, Greece
Received 2018-8-11; Accepted 2018-12-9; Published 2019-1-25
Abstract
Current statistics indicate that hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third leading cause of cancer-related death. Major predisposing conditions are hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. To date treatment approach includes liver transplantation, surgical resection and or ablation, however; recurrence, metastasis, and mortality still remains high. Therefore, alternative treatments such as gene therapy is increasingly being considered as a feasible proposal. In this mini review we will focus on novel data of the past 10 years on the subject of gene therapy and hepatocellular carcinoma.
Keywords: vectors, hepatocellular carcinoma, gene therapy
Introduction
Multimodality treatment with surgery, radiotherapy and chemotherapy still remains the cornerstone of cancer treatment. Based on the stage during diagnosis neo-adjuvant or adjuvant treatment is applicable for several cancer types. Unfortunately chemotherapy and radiotherapy have adverse effects and in several situations the patients` treatment has to be postponed until these fade away. Most common adverse effects arise from bone marrow suppression [1]. In several cases bone marrow suppression requires hospitalization [2, 3]. Therefore novel routes of administration and treatments have been investigated for many types of cancer, with the main concept being the local treatment [4-14]. Chemotherapy is administered in most cases intravenously and in some cases orally. However, breakthroughs in revealing the genome mutations of tumors have identified specific mutated populations where targeted therapy can be administered orally [15-17]. Multiple genetic mutations and pathways are currently being investigated for several cancer types of liver cancer [18]. In these populations certain pathways which are overexpressed have been targeted with novel drug formulations [19-21]. Pharmacogenetics is currently the tip of the arrow for novel drug, design, development and therapy [22]. Unfortunately there are several different genetic profiles with very few patients for each mutation. In this group of patients new mutations can occur after targeted treatment, but again new inhibitors can be administered [23]. Unfortunately there are resistance mechanisms that tumors develop therefore new strategies are used to sensitize tumors to chemotherapy and radiotherapy [24]. Another less toxic therapy that is currently being investigated is gene therapy. Suicide gene therapy (a type of gene therapy) has the ability to convert a non-toxic drug which penetrates the tumor, to cytotoxic. The conversion of the pro-drug to active drug takes action inside the tumor with the help of an administered viral or bacterial gene. Most importantly the normal cells are not affected [25-27]. To date suicide gene therapy has been investigated in: a) liver [9, 28, 29], b) colon [8, 30, 31], c) neuroendocrine [32], d) lung [33, 34], e) medulloblastomas [35], f) spinal cord tumors [36], g) prostate [37], h) breast [38, 39], i) bladder [40], j) brain [41], k) head and neck [42], l) gliomas [43-45] and m) sarcomas [46]. It has been observed that suicide gene therapy is efficient in chemotherapy resistant cancer cell lines [47] and can enhance radiotherapy [48]. In a recent study it was observed that micrometastasis were efficiently controlled with suicide gene therapy [49]. Moreover; suicide gene therapy is being explored by using technology such as nanoparticles to efficiently penetrate any tumor microenvironment [50-52] (Figure 1). In the current mini review article we will focus on hepatocellular carcinoma and the current knowledge of the last 10 years.
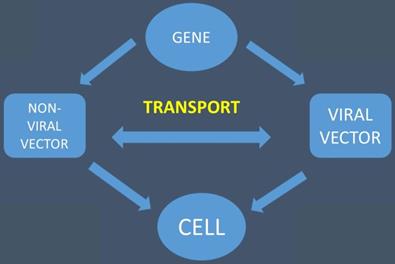
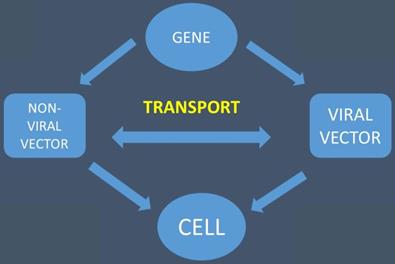
The gene is transported with the help of viral and non-viral vectors to the cell
Research Strategies
We performed an electronic article search through PubMed, Google Scholar, Medscape, and Scopus databases, using combinations of the following keywords: cancer gene therapy, bystander effect, suicide gene therapy, gene therapy, suicide gene therapies strategies, vectors for gene therapy. All types of articles (randomized controlled trials, clinical observational cohort studies, review articles, case reports) were included. Selected references from identified articles were searched for further consideration, without language limitation. We focused mostly on data published in the past 10 years.
Hepatocellular
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and currently ranks third among the most prevalent deadly cancers in the world [53]. The incidence of HCC will rise in the next years due to the increasing prevalence of hepatitis C virus (HCV) in Europe, North America and Japan. Moreover; the risk for HCC development is now known to be age-dependent [54]. To date surgery and transplantation remains the only potentially curative modality, however; the recurrence rate is high and there is poor long-term survival [55]. Furthermore, radiotherapy (RT) is an additional treatment modality to treat hepatoma patients [56]. However, again RT treatment in HCC patients suffer from recurrence problems due to radio-resistance acquisition. Radio-resistance still remains a serious impediment to successful treatment of HCC patients [57].
Studies
In the study by Sia KC et al. [9] the hepatocellular cancer cell line was cultured to evaluate a suicide gene therapy model. The herpes simplex virus type 1 (HSV-1) amplicon viral vector was coupled with yCD 5-FC and was administered. It was observed that HCC 26-1004 tumor xenograf expressed high levels of y-CD genes with non-invasive imaging. The firefly luciferase reporter gene is a valuable tool to monitor the activity and bio distribution of the therapeutic gene expression, however; it cannot be used in clinical trials as it is not bio-compatible. In the same study it was proposed by the authors radiation to be used along with 5-FU as it known to be a radiosensitizer agent [58]. In the study by Lin L et al. [59] it was observed that γ-Glutamylcysteine synthetase (γ‑GCSh) could represent a prime target for overcoming resistance of anticancer drugs and radiotherapy for HCC cells. In the study by Luo X et al. [60] it was observed that stress-induced phosphoprotein 1 (STIP1) is up-regulated in the HCC tissues. STIP1 plays an oncogenic role in the progression of HCC, and it was suggested that STIP1 might be a therapeutic target. In the study by Xu D et al. [61] the results indicated that the promoter hypermethylation of GNAO1 might play an important role in HCC. GNAO1 might also be used as a biomarker for hepatocellular diagnosis and a future targeted therapy. In the study by Ceballos MP et al. [62] Sirtuins (SIRTs) 1 and 2 deacetylases were overexpressed in hepatocellular carcinoma and were associated with tumoral progression and multidrug resistance. It was supported that downregulation of the expression of P-gp and MRP3 supports the potential application of SIRTs 1 and 2 inhibitions in combination with conventional chemotherapy. In the study by Zhuang H et al. [63] it was reported that Glycine decarboxylase (GLDC), an oxidoreductase, plays an important role in amino acid metabolism. The data of the study indicate that GLDC downregulation decreases ROS-mediated ubiquitination of cofilin and as a result enhances hepatocellular progression. In the study by Qiu Z. et. al. [64] the findings of the study indicated that urolithin A exerted an antiproliferative effect by regulating the Lin28a/let-7a axis and may be a potential supplement for HBV-infected HCC therapy. In the study by Liu X et al. [65] the findings suggested that Stellerachamaejasme L (ESC) regressed growth and metastasis of human hepatocellular carcinoma, by downregulating microRNAs expression. In the study by Yang B. et al. [66] experiments and clinical samples demonstrated a dynamic network biomarker (DNB) with calmodulin-like protein 3 (CALML3) reduced pulmonary metastasis in liver cancer. The loss of calmodulin-like protein 3 predicted shorter overall survival and disease free survival in postoperative HCC patients. Therefore it could be used as a prognostic biomarker and a future therapy target in HCC. In the study by Sheng J et al. [67] it was reported that various chemotherapeutic drugs for HCC treatment can increase autophagic flux of HCC cells. Moreover; it was related with enhancing drug resistance and promoting cell survival. It is known that most hepatocellular patients are insensitive to chemotherapeutic drugs, and resistance usually develops after a few sessions of chemotherapy treatment. Autophagy induction is a frequent response of HCC cells to chemotherapeutic drugs and induces acquired resistance. In the study by Ye Y et al. [68] insulin‑like growth factor receptor‑1 (IGF1R) was identified as a direct target gene of miR‑495 in hepatocellular carcinoma. Insulin‑like growth factor receptor‑1 was upregulated in hepatocellular tissues and negatively correlated with miR‑495 expression level. The upregulation of insulin‑like growth factor receptor‑1 rescued the miR‑495‑induced tumor‑suppressive roles in hepatocellular carcinoma cell proliferation and invasion. Finally, restored miR‑495 expression and inactivated the protein kinase B and extracellular regulated protein kinase signaling pathway. These results indicate that miR‑495 may be a novel therapeutic target for patients with hepatocellular carcinoma. In the study by He RQ et al. [69] the potential role of miR-23b-3p in hepatocellular carcinoma tumorigenesis and progression was elucidated. Moreover; miR-23b-3p it was suggested that it may act as a predictor of HCC. In the study by Xia Y et al. [70] the HA-Se-PEI@siRNA was internalized into the HepG2 cell mainly in a clathrin-mediated endocytosis manner. The treatment with HA-Se-PEI@siRNA resulted in greater antitumor efficacy compared with the Se-PEI@siRNA in vitro and in vivo. Moreover; HA-Se-PEI@siRNA was almost no toxic to kidneys, lungs, heart and liver of mice. In the study by Wang L et al. [71] the results of the study indicated that alpha induced protein 8 like 2 (TIPE‑2) acts as an inhibitor of HCC cell growth and promotes apoptosis. Alpha induced protein 8 like 2 may inhibit the metastasis‑associated PI3K/AKT signaling cascade and may downregulate the tumor cell cycle. These findings provided the mechanism by which TIPE‑2 promotes apoptosis of hepatocellular carcinoma. In the study by Liu J et al. [72] it was revealed that the growth of tumor was significantly suppressed after the transfection of T-cell immunoglobulin and mucin-domain containing-3. In the presence of T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), the proliferation of splenocytes and cytolysis in the early phase of tumor development was significantly enhanced. Moreover; the antitumor effect was further improved by the synergistic effect of Tim‑3 with Transporter associated with Antigen Processing 1 (TAP1). Therefore, the membrane‑type Tim‑3 is an effective immunoregulator and enhances antitumor immune response. In the study by Bai G et al. [73] cyclin dependent kinase 1 (CDK1), NDC80, cyclin A2 (CCNA2) and rac GTPase activating protein 1 (RACGAP1) were shown to be targeted by the HCV nonstructural proteins NS5A, NS3 and NS5B, respectively. It was observed that the four genes perform an intermediary role between the HCV viral proteins and the dysfunctional module in the HCV key genes interaction network. In the study by Xue Y et al. [74] the results of the study suggested that the shRNA‑mediated knockdown of Frizzled‑7 (FZD7) induced apoptosis of HCC lines through the inhibition of nuclear factor‑κB (NF‑κB). In addition, the transforming growth factor (TGF‑β)/Smad signaling pathway appeared to participate in the underlying mechanism of FZD7 in hepatocellular carcinoma cell lines. In the study by Li M et al. [75] results indicated that deguelin could inhibit hepatocellular carcinoma cell lines through suppression of angiogenesis and reduce proangiogenic factors in cancer cells. In the study by Dhanasekaran R et al. [76] global gene expression profiling revealed engagement of miR-17 target genes and inhibition of key transcriptional programs of family of regulator genes and proto-oncogenes that code for transcription factors (three related human genes: c-myc, l-myc, and n-myc. c-myc called MYC). Therefore, anti-miR-17 is an effective therapy for MYC-driven hepatocellular carcinoma cell lines. In the study by Ogura S et al. [77] the data indicated that FoxM1 links the mevalonate pathway to oncogenic signals in hepatocellular carcinoma cell lines. The results indicated that a novel therapeutic approach to inhibit FoxM1 by targeting the mevalonate pathway for hepatocellular carcinoma cell lines is feasible.
Discussion
In order for gene therapy to work specific vectors or pro-drugs have to be chosen for each cancer type. Novel vectors and transporters of genes are in need. Currently the thymidine-active mutant of dCK, dCK.DM.S74E was created which activates multiple pro-drugs such as; BVdU, LdUNAs and LdT. It has been observed that the system has the ability to sensitize and re-sensitize tumors to chemotherapeutic agents. Moreover; it was observed that it can activate more than one pro-drug simultaneously and prevents multidrug resistance without any additional side effects [78]. Another method to enhance gene therapy is local application to the tumor site [6, 79]. There are cases where loco-regional administration is not possible and therefore systematic administration has to be chosen. We need drugs that have a sustain release effect. The concept relies on the “stealth” ability of several molecules to circulate within the blood stream without being detected and have a sustain release gene expression effect.[80, 81] In the study by Zhao Y et al. the neural stem cells (NSCs) derived from hairy and enhancer of split-1 (HES1) human embryonic stem cell line had the ability to migrate from the injection site. In order for the administration system to work a baculovirus vector was used to insert the HSV-tk suicide gene into the cells. Moreover; ganciclovir was co-administered in order for an amount of concentration to be present locally. Indeed the transgene expression was present for three weeks [43]. The same concept was also applied with mesenchymal stem cells (MSC) in a hepatocellular carcinoma cell lines model [82]. In the study by Wang C et al. [41] investigated neural stem cells (NSCs) (F3) as dual suicide gene therapy with Cytosine Deaminase (CD) and Thymidine Kinase (TK) creating the NSC-F3.CD-TK. Enhanced antitumor activity against lung cancer metastasis in comparison to single suicide gene therapy was observed. In another study lung cancer cell lines were used along with a carcinoembryonic antigen (CEA) promoter with Thymidine Kinase and Cytosine Deaminase constructing the pCEA-TK/CD [34]. Dual suicide gene therapy has been investigated with surviving promoter Ad-survivin/GFP and Ad-survivin/CD/TK and as anticipated higher efficiency was observed compared to single suicide gene therapy [83]. Moreover; combination suicide gene therapy has been investigated with (VSV)-ΜΔ51-expressing (CD::UPRT)-5FC in four different cancer cell lines (Prostate PC3, Breast MCF7, TSA mammary, Adenocarcinoma, B-Lymphoma Karpas-422, and Melanoma B16-F10) and as a result increased tumor oncolysis was observed [84]. The combination treatment was more effective against the parental mammary adenocarcinoma (TSA). Unfortunately dual gene therapy is not applicable for all cancer types. In the study by Tang Q et al. [52] local treatment was enhanced in a human hepatic cancer cell line model by intratumoral administration of KDR-TK and Alpha-fetoprotein -Thymidine Kinase-Luciferase Knockin Mice (AFP-TK) with microbubble contrast agent prior to ultrasound treatment. The pro-drugs 5-Fluoracil (5-FC) and ganciclovir (GCV) were administered after the intratumoral therapy. Between the two groups there was no difference in the antitumor activity. The time of the pro-drug administration is crucial for the efficiency of the treatment as the pro-drug has to be already diffused within the target tissue for the administered gene therapy to be effective. In the study by Duan X et al. [50] gene transfection of the novel cationic self-assembled DOTAP and MPEG-PCL hybrid micelles (DMP) was investigated. The new transport had less toxicity compared to the polymer Polyethyleneimine (PEI) with 25kDa. In the present study DMP delivered efficiently the urvivin-T34 gene (S-T34A) to treat C-26 colon cancer cell lines (CLC). Nanoparticles have been used previously in other gene transfection studies [85, 86]. To date there are very few studies with suicide gene therapy in clinical trials and every effort towards [6, 87-99]. Recently a clinical trial for prostate cancer was published and others in extensive stage followed [37, 96]. In a recent study the suicide gene TK.007, was used and demonstrated efficiency in several cancer cell lines (G62 human glioblastoma cell line, SW620 human colorectal adenocarcinoma cell line, A549 human lung carcinoma, and IPC298 human melanoma cell line) [100]. It was observed that TK.007 had higher gene transfection in comparison to HSV-tk with lower doses of ganciclovir. The spliceosome-mediated RNA trans-splicing technology has the ability to replace a tumor-specific transcript with one encoding a cell death-inducing peptide/toxin. This technology enhances the gene transfection. In another study by Gruber C et al. [101] the efficiency of 3' pre-trans-splicing molecules (PTM) was investigated against highly malignant tumors. The group of Di Stasi et al. [102] developed a new system that targets the inducible caspase 9 (iCasp9) gene. Firstly it was developed for children who developed graft-vs.-host disease (GVHD) by donor lymphocytes. The process was fortunately reversed with the novel therapy. Receptors on living cells have been used as targets for gene therapy, such as; vascular endothelial growth factor (VEGF) [51, 85] and carcino-embryonic antigen (CEA) [34]. Moreover; there are other receptors that can be targeted for HCC gene therapy such as; a) cluster of differentiation (CD44s) [103], b) epidermal growth factor receptor (EGFR) [104], c) folate receptor (FR) [105], d) stage specific embryonic antigen 4 [106], e) cluster of differentiation [107], f) transferrin receptor (TfR) or cluster differentiation 71 (CD71) [108], g) mucins [109], and h) tumor resistance antigen 1-60 (TRA-1-60) [110] (Table 1). In the future we want small molecules that can be used as carriers for gene therapy and have a sustain release effect either for local treatment or systemic treatment. Additional modalities such as; radiotherapy could be added prior to local treatment.
Pathways and Promoters
| Targeted Pathways |
| -Epidermal Growth Factor Receptors |
| -Vascular Endothelial Growth Factor |
| -Carcino-embryonic antigen |
| -Transferrin receptor |
| -Mucins |
| -Cluster differentiation 44 |
| -Cluster differentiation 133 |
| -Stage specific embryonic antigen 4 |
| -Tumor resistance antigen 1-60 |
| Promoters |
| -Epidermal Growth Factor Receptors |
| -Transferrin receptor |
| -Carcino-embryonic antigen |
| -Prostate specific antigen |
| -Telomerase-hTERT |
| -Cycloxiganase |
| -Cytokeratin 18-19 |
Competing Interests
The authors have declared that no competing interest exists.
References
1. Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT. et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155-66
2. Kuwabara H, Fushimi K. The impact of a new payment system with case-mix measurement on hospital practices for breast cancer patients in Japan. Health Policy. 2009;92:65-72
3. Lee MK, Dodson TB, Nalliah RP, Karimbux NY, Allareddy V. Nine-year trend analysis of hospitalizations attributed to oral and oropharyngeal cancers in the United States. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013
4. Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP. et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551-72
5. Zarogoulidis P, Eleftheriadou E, Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T. et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investigational new drugs. 2012;30:1628-40
6. Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, Goldberg EP, Galaktidou G, Kontakiotis T. et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther. 2012;19:593-600
7. Darwiche K, Zarogoulidis P, Karamanos NK, Domvri K, Chatzaki E, Constantinidis TC. et al. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology. Future Oncol. 2013;9:505-25
8. Mader RM, Kalipciyan M, Ohana P, Hochberg A, Steger GG. Suicide activation in a 5-fluorouracil resistant colon cancer model in vitro. Int J Clin Pharmacol Ther. 2011;49:69-70
9. Sia KC, Huynh H, Chinnasamy N, Hui KM, Lam PY. Suicidal gene therapy in the effective control of primary human hepatocellular carcinoma as monitored by noninvasive bioimaging. Gene Ther. 2012;19:532-42
10. Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008;61:1-12
11. Zhibing W, Qinghua D, Shenglin M, Ke Z, Kan W, Xiadong L. et al. Clinical study of cisplatin hyperthermic intraperitoneal perfusion chemotherapy in combination with docetaxel, 5-flourouracil and leucovorin intravenous chemotherapy for the treatment of advanced-stage gastric carcinoma. Hepatogastroenterology. 2013 60
12. Porpodis K, Karanikas M, Zarogoulidis P, Kontakiotis T, Mitrakas A, Esebidis A. et al. A case of typical pulmonary carcinoid tumor treated with bronchoscopic therapy followed by lobectomy. J Multidiscip Healthc. 2012;5:47-51
13. Podolska K, Stachurska A, Hajdukiewicz K, Malecki M. Gene therapy prospects-intranasal delivery of therapeutic genes. Adv Clin Exp Med. 2012;21:525-34
14. Domvri K, Zarogoulidis P, Porpodis K, Koffa M, Lambropoulou M, Kakolyris S. et al. Gene therapy in liver diseases: state-of-the-art and future perspectives. Curr Gene Ther. 2012;12:463-83
15. Tsoulos N, Papadopoulou E, Metaxa-Mariatou V, Tsaousis G, Efstathiadou C, Tounta G. et al. Tumor molecular profiling of NSCLC patients using next generation sequencing. Oncology reports. 2017;38:3419-29
16. Domvri K, Zarogoulidis P, Darwiche K, Browning RF, Li Q, Turner JF. et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. Journal of Cancer. 2013;4:736-54
17. Domvri K, Darwiche K, Zarogoulidis P, Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Translational lung cancer research. 2013;2:256-8
18. Weng L, Zhang L, Peng Y, Huang RS. Pharmacogenetics and pharmacogenomics: a bridge to individualized cancer therapy. Pharmacogenomics. 2013;14:315-24
19. Cervino AR, Burei M, Mansi L, Evangelista L. Molecular pathways and molecular imaging in breast cancer: An update. Nucl Med Biol. 2013
20. Matthaios D, Zarogoulidis P, Balgouranidou I, Chatzaki E, Kakolyris S. Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology. 2011;81:259-72
21. Machairiotis N, Kougioumtzi I, Zarogoulidis P, Stylianaki A, Tsimogiannis K, Katsikogiannis N. Gastrointestinal stromal tumor mesenchymal neoplasms: the offspring that choose the wrong path. J Multidiscip Healthc. 2013;6:127-31
22. Trent RJ, Cheong PL, Chua EW, Kennedy MA. Progressing the utilisation of pharmacogenetics and pharmacogenomics into clinical care. Pathology. 2013
23. Zarogoulidis P, Rapti A, Sardeli C, Chinelis P, Athanasiadou A, Paraskevaidou K. et al. Re-biopsy after relapse of targeted therapy. T790M after epidermal growth factor mutation, where and why based on a case series. Respiratory medicine case reports. 2017;21:171-5
24. Vachani A, Moon E, Wakeam E, Haas AR, Sterman DH, Albelda SM. Gene therapy for lung neoplasms. Clin Chest Med. 2011;32:865-85
25. Duarte S, Carle G, Faneca H, de Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324:160-70
26. Malecki M. Frontiers in Suicide Gene Therapy of Cancer. J Genet Syndr Gene Ther. 2012. 2012
27. Morgan RA. Live and let die: a new suicide gene therapy moves to the clinic. Mol Ther. 2012;20:11-3
28. Niu HX, Du T, Xu ZF, Zhang XK, Wang RG. Role of wild type p53 and double suicide genes in interventional therapy of liver cancer in rabbits. Acta Cir Bras. 2012;27:522-8
29. Marukawa Y, Nakamoto Y, Kakinoki K, Tsuchiyama T, Iida N, Kagaya T. et al. Membrane-bound form of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy in a model of hepatocellular carcinoma. Cancer Gene Ther. 2012;19:312-9
30. Ahn YH, Yi H, Shin JY, Lee KD, Shin SP, Lee SJ. et al. STAT3 silencing enhances the efficacy of the HSV.tk suicide gene in gastrointestinal cancer therapy. Clin Exp Metastasis. 2012;29:359-69
31. Finzi L, Kraemer A, Capron C, Noullet S, Goere D, Penna C. et al. Improved retroviral suicide gene transfer in colon cancer cell lines after cell synchronization with methotrexate. J Exp Clin Cancer Res. 2011;30:92
32. Akerstrom V, Chen C, Lan MS, Breslin MB. Adenoviral insulinoma-associated protein 1 promoter-driven suicide gene therapy with enhanced selectivity for treatment of neuroendocrine cancers. Ochsner J. 2013;13:91-9
33. Cramer F, Christensen CL, Poulsen TT, Badding MA, Dean DA, Poulsen HS. Insertion of a nuclear factor kappa B DNA nuclear-targeting sequence potentiates suicide gene therapy efficacy in lung cancer cell lines. Cancer Gene Ther. 2012;19:675-83
34. Qiu Y, Peng GL, Liu QC, Li FL, Zou XS, He JX. Selective killing of lung cancer cells using carcinoembryonic antigen promoter and double suicide genes, thymidine kinase and cytosine deaminase (pCEA-TK/CD). Cancer Lett. 2012;316:31-8
35. Li S, Gao Y, Pu K, Ma L, Song X, Liu Y. All-trans retinoic acid enhances bystander effect of suicide-gene therapy against medulloblastomas. Neurosci Lett. 2011;503:115-9
36. Won YW, Kim KM, An SS, Lee M, Ha Y, Kim YH. Suicide gene therapy using reducible poly (oligo-D-arginine) for the treatment of spinal cord tumors. Biomaterials. 2011;32:9766-75
37. Lu M, Freytag SO, Stricker H, Kim JH, Barton K, Movsas B. Adaptive seamless design for an efficacy trial of replication-competent adenovirus-mediated suicide gene therapy and radiation in newly-diagnosed prostate cancer (ReCAP Trial). Contemp Clin Trials. 2011;32:453-60
38. Ma S, Zhao L, Zhu Z, Liu Q, Xu H, Johansson M. et al. The multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster as a therapeutic suicide gene of breast cancer cells. J Gene Med. 2011;13:305-11
39. Yi BR, Choi KJ, Kim SU, Choi KC. Therapeutic potential of stem cells expressing suicide genes that selectively target human breast cancer cells: evidence that they exert tumoricidal effects via tumor tropism (review). Int J Oncol. 2012;41:798-804
40. Yin X, Yu B, Tang Z, He B, Ren J, Xiao X. et al. Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther. 2013;20:77-81
41. Wang C, Natsume A, Lee HJ, Motomura K, Nishimira Y, Ohno M. et al. Neural stem cell-based dual suicide gene delivery for metastatic brain tumors. Cancer Gene Ther. 2012;19:796-801
42. Schmidt M, Gruensfelder P, Roller J, Hagen R. Suicide gene therapy in head and neck carcinoma cells: an in vitro study. Int J Mol Med. 2011;27:591-7
43. Zhao Y, Lam DH, Yang J, Lin J, Tham CK, Ng WH. et al. Targeted suicide gene therapy for glioma using human embryonic stem cell-derived neural stem cells genetically modified by baculoviral vectors. Gene Ther. 2012;19:189-200
44. Kosaka H, Ichikawa T, Kurozumi K, Kambara H, Inoue S, Maruo T. et al. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572-8
45. Cottin S, Gould PV, Cantin L, Caruso M. Gap junctions in human glioblastomas: implications for suicide gene therapy. Cancer Gene Ther. 2011;18:674-81
46. Finocchiaro LM, Villaverde MS, Gil-Cardeza ML, Riveros MD, Glikin GC. Cytokine-enhanced vaccine and interferon-beta plus suicide gene as combined therapy for spontaneous canine sarcomas. Res Vet Sci. 2011;91:230-4
47. Michaelsen SR, Christensen CL, Sehested M, Cramer F, Poulsen TT, Patterson AV. et al. Single agent- and combination treatment with two targeted suicide gene therapy systems is effective in chemoresistant small cell lung cancer cells. J Gene Med. 2012;14:445-58
48. Sun X, Xing L, Deng X, Hsiao HT, Manami A, Koutcher JA. et al. Hypoxia targeted bifunctional suicide gene expression enhances radiotherapy in vitro and in vivo. Radiother Oncol. 2012;105:57-63
49. Kakinoki K, Nakamoto Y, Kagaya T, Tsuchiyama T, Sakai Y, Nakahama T. et al. Prevention of intrahepatic metastasis of liver cancer by suicide gene therapy and chemokine ligand 2/monocyte chemoattractant protein-1 delivery in mice. J Gene Med. 2010;12:1002-13
50. Duan X, Wang P, Men K, Gao X, Huang M, Gou M. et al. Treating colon cancer with a suicide gene delivered by self-assembled cationic MPEG-PCL micelles. Nanoscale. 2012;4:2400-7
51. Leng A, Yang J, Liu T, Cui J, Li XH, Zhu Y. et al. Nanoparticle-delivered VEGF-silencing cassette and suicide gene expression cassettes inhibit colon carcinoma growth in vitro and in vivo. Tumour Biol. 2011;32:1103-11
52. Tang Q, He X, Liao H, He L, Wang Y, Zhou D. et al. Ultrasound microbubble contrast agent-mediated suicide gene transfection in the treatment of hepatic cancer. Oncol Lett. 2012;4:970-2
53. Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A. et al. Cancer statistics, 2005. CA: a cancer journal for clinicians. 2005;55:10-30
54. Roberts LR, Gores GJ. Emerging drugs for hepatocellular carcinoma. Expert opinion on emerging drugs. 2006;11:469-87
55. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nature genetics. 2002;31:339-46
56. Meng J, Cao LM, Hu CP, Zhen ZY. [Establishment of a lung adenocarcinoma cell line stably expressing small interfering RNA targeting hepatoma-derived growth factor and detection of interference effect]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2009;29:2233-6
57. Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX. et al. Intratumoral regulatory T cells with higher prevalence and more suppressive activity in hepatocellular carcinoma patients. Journal of gastroenterology and hepatology. 2013;28:1555-64
58. Lawrence TS, Davis MA, Maybaum J. Dependence of 5-fluorouracil-mediated radiosensitization on DNA-directed effects. Int J Radiat Oncol Biol Phys. 1994;29:519-23
59. Lin LC, Chen CF, Ho CT, Liu JJ, Liu TZ, Chern CL. gamma-Glutamylcysteine synthetase (gamma-GCS) as a target for overcoming chemo- and radio-resistance of human hepatocellular carcinoma cells. Life sciences. 2018;198:25-31
60. Luo X, Liu Y, Ma S, Liu L, Xie R, Li M. et al. STIP1 is over-expressed in hepatocellular carcinoma and promotes the growth and migration of cancer cells. Gene. 2018;662:110-7
61. Xu D, Du M, Zhang J, Xiong P, Li W, Zhang H. et al. DNMT1 mediated promoter methylation of GNAO1 in hepatoma carcinoma cells. Gene. 2018;665:67-73
62. Ceballos MP, Decandido G, Quiroga AD, Comanzo CG, Livore VI, Lorenzetti F. et al. Inhibition of sirtuins 1 and 2 impairs cell survival and migration and modulates the expression of P-glycoprotein and MRP3 in hepatocellular carcinoma cell lines. Toxicology letters. 2018;289:63-74
63. Zhuang H, Li Q, Zhang X, Ma X, Wang Z, Liu Y. et al. Downregulation of glycine decarboxylase enhanced cofilin-mediated migration in hepatocellular carcinoma cells. Free radical biology & medicine. 2018;120:1-12
64. Qiu Z, Zhou J, Zhang C, Cheng Y, Hu J, Zheng G. Antiproliferative effect of urolithin A, the ellagic acid-derived colonic metabolite, on hepatocellular carcinoma HepG2.2.15 cells by targeting Lin28a/let-7a axis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2018;51:e7220
65. Liu X, Wang S, Xu J, Kou B, Chen D, Wang Y. et al. Extract of Stellerachamaejasme L(ESC) inhibits growth and metastasis of human hepatocellular carcinoma via regulating microRNA expression. BMC complementary and alternative medicine. 2018;18:99
66. Yang B, Li M, Tang W, Liu W, Zhang S, Chen L. et al. Dynamic network biomarker indicates pulmonary metastasis at the tipping point of hepatocellular carcinoma. Nature communications. 2018;9:678
67. Sheng J, Qin H, Zhang K, Li B, Zhang X. Targeting autophagy in chemotherapy-resistant of hepatocellular carcinoma. American journal of cancer research. 2018;8:354-65
68. Ye Y, Zhuang J, Wang G, He S, Zhang S, Wang G. et al. MicroRNA-495 suppresses cell proliferation and invasion of hepatocellular carcinoma by directly targeting insulin-like growth factor receptor-1. Experimental and therapeutic medicine. 2018;15:1150-8
69. He RQ, Wu PR, Xiang XL, Yang X, Liang HW, Qiu XH. et al. Downregulated miR-23b-3p expression acts as a predictor of hepatocellular carcinoma progression: A study based on public data and RT-qPCR verification. International journal of molecular medicine. 2018;41:2813-31
70. Xia Y, Guo M, Xu T, Li Y, Wang C, Lin Z. et al. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. International journal of nanomedicine. 2018;13:1539-52
71. Wang L, Chen C, Feng S, Tian J. TIPE2 suppresses growth and aggressiveness of hepatocellular carcinoma cells through downregulation of the phosphoinositide 3kinase/AKT signaling pathway. Molecular medicine reports. 2018;17:7017-26
72. Liu J, Sun H, Shen D, Wang M, Wen Z. Antitumor effect of membrane-type Tim-3 on hepatocellular carcinoma Hepa1-6 cells of ICR mice. Oncology letters. 2018;15:2631-4
73. Bai G, Zheng W, Ma W. Identification and functional analysis of a core gene module associated with hepatitis C virus-induced human hepatocellular carcinoma progression. Oncology letters. 2018;15:6815-24
74. Xue Y, Chen C, Xu W, Xu H, Zheng J, Gu Y. Downregulation of Frizzled-7 induces the apoptosis of hepatocellular carcinoma cells through inhibition of NF-kappaB. Oncology letters. 2018;15:7693-701
75. Li M, Yu X, Li W, Liu T, Deng G, Liu W. et al. Deguelin suppresses angiogenesis in human hepatocellular carcinoma by targeting HGF-c-Met pathway. Oncotarget. 2018;9:152-66
76. Dhanasekaran R, Gabay-Ryan M, Baylot V, Lai I, Mosley A, Huang X. et al. Anti-miR-17 therapy delays tumorigenesis in MYC-driven hepatocellular carcinoma (HCC). Oncotarget. 2018;9:5517-28
77. Ogura S, Yoshida Y, Kurahashi T, Egawa M, Furuta K, Kiso S. et al. Targeting the mevalonate pathway is a novel therapeutic approach to inhibit oncogenic FoxM1 transcription factor in human hepatocellular carcinoma. Oncotarget. 2018;9:21022-35
78. Neschadim A, Wang JC, Lavie A, Medin JA. Bystander killing of malignant cells via the delivery of engineered thymidine-active deoxycytidine kinase for suicide gene therapy of cancer. Cancer Gene Ther. 2012;19:320-7
79. Amano S, Gu C, Koizumi S, Tokuyama T, Namba H. Tumoricidal bystander effect in the suicide gene therapy using mesenchymal stem cells does not injure normal brain tissues. Cancer Lett. 2011;306:99-105
80. Karponi G, Zogas N, Domvri K, Zarogoulidis P, Trakada G, Roumeliotis S. et al. Prospects of gene therapy for pulmonary diseases: progress and limitations. Medicinal chemistry. 2017 doi:10.2174/1573406413666170209122131
81. Zarogoulidis P, Darwiche K, Hohenforst-Schmidt W, Huang H, Li Q, Freitag L. et al. Inhaled gene therapy in lung cancer: proof-of-concept for nano-oncology and nanobiotechnology in the management of lung cancer. Future oncology. 2013;9:1171-94
82. Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F. et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg. 2011;254:767-74 discussion 74-5
83. Luo XR, Li JS, Niu Y, Miao L. Adenovirus-mediated double suicide gene selectively kills gastric cancer cells. Asian Pac J Cancer Prev. 2012;13:781-4
84. Leveille S, Samuel S, Goulet ML, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435-43
85. Liu T, Ye L, He Y, Chen X, Peng J, Zhang X. et al. Combination gene therapy using VEGF-shRNA and fusion suicide gene yCDglyTK inhibits gastric carcinoma growth. Exp Mol Pathol. 2011;91:745-52
86. Li J, Zhang G, Liu T, Gu H, Yan L, Chen B. Construction of a novel vector expressing the fusion suicide gene yCDglyTK and hTERT-shRNA and its antitumor effects. Exp Ther Med. 2012;4:442-8
87. Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A. et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456-66
88. Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT. et al. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther. 2010;18:852-60
89. Dong M, Li X, Hong LJ, Xie R, Zhao HL, Li K. et al. Advanced malignant pleural or peritoneal effusion in patients treated with recombinant adenovirus p53 injection plus cisplatin. J Int Med Res. 2008;36:1273-8
90. Zhao WZ, Wang JK, Li W, Zhang XL. [Clinical research on recombinant human Ad-p53 injection combined with cisplatin in treatment of malignant pleural effusion induced by lung cancer]. Ai Zheng. 2009;28:1324-7
91. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389-401
92. Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G. et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479-87
93. Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F. et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834-40
94. Xu F, Li S, Li XL, Guo Y, Zou BY, Xu R. et al. Phase I and biodistribution study of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene and ganciclovir administration in patients with head and neck cancer and other malignant tumors. Cancer Gene Ther. 2009;16:723-30
95. Li N, Zhou J, Weng D, Zhang C, Li L, Wang B. et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2007;13:5847-54
96. Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J. et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016-23
97. Pandha HS, Martin LA, Rigg A, Hurst HC, Stamp GW, Sikora K. et al. Genetic prodrug activation therapy for breast cancer: A phase I clinical trial of erbB-2-directed suicide gene expression. J Clin Oncol. 1999;17:2180-9
98. Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J. et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497-506
99. Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C. et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737-44
100. Preuss E, Muik A, Weber K, Otte J, von Laer D, Fehse B. Cancer suicide gene therapy with TK.007: superior killing efficiency and bystander effect. J Mol Med (Berl). 2011;89:1113-24 doi:10.1007/s00109-011-0777-8
101. Gruber C, Gratz IK, Murauer EM, Mayr E, Koller U, Bruckner-Tuderman L. et al. Spliceosome-mediated RNA trans-splicing facilitates targeted delivery of suicide genes to cancer cells. Mol Cancer Ther. 2011;10:233-41
102. Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673-83
103. Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567-79
104. Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087-98
105. Duarte S, Faneca H, de Lima MC. Non-covalent association of folate to lipoplexes: a promising strategy to improve gene delivery in the presence of serum. J Control Release. 2011;149:264-72
106. Malecki M, Anderson M, Beauchaine M, Seo S, Tambokan X. TRA-1-60(+), SSEA-4(+), Oct4A(+), Nanog(+) Clones of Pluripotent Stem Cells in the Embryonal Carcinomas of the Ovaries. J Stem Cell Res Ther. 2012 2
107. Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ, Wang H. et al. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012;10:85
108. Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR. et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119:283-93
109. Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472-81
110. Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005;33:1526-30
Author contact
![]() Corresponding author: Paul Zarogoulidis M.D, Ph.D, Pulmonary-Oncology Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom
Corresponding author: Paul Zarogoulidis M.D, Ph.D, Pulmonary-Oncology Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom