J Biomed 2018; 3:64-67. doi:10.7150/jbm.25976 This volume Cite
Review
MicroRNA Expression Patterns Responsible for Inducing Chemo-Resistance in Ovarian Cancer
1. Department of Biological and Environmental Sciences, University of Houston-Clear Lake, 2700 Bay Area Blvd., Houston, Texas 77058, USA.
2. School of Natural Sciences, University of California Merced, 5200 North Lake Rd. Merced, CA 95343
Received 2018-3-9; Accepted 2018-6-9; Published 2018-7-1
Abstract
Despite significant progress in our understanding of the pathophysiology of cancer as a whole, ovarian cancer is still remains as one of the most intractable forms of the disease with only 30% cure rate with the conventional therapy. It is also one of most common gynecologic cancer among women. One histologic variety is the epithelial ovarian cancer that constitutes more than 90% of the cases; also develop resistance to chemotherapeutic agents. Recent studies have demonstrated the role of micro RNA (miRNA) in the evolution and progression of ovarian cancer. Furthermore, aberrant expression of miRNA has been implicated in the development of resistance to chemotherapy in such cases. This review compiles recent advances in the role of miRNA in chemoresistant ovarian cancer and approaches to tackle the problem and design therapy that is more effective.
Keywords: chemo-resistance, microRNA, ovarian cancer
Since the first reporting in 1993 (1), miRNA has been found to be involved in many fundamental cellular processes including differentiation, proliferation, apoptosis and metabolism (2) by altering the translational output through imperfect base pairing with the target mRNA (3). Aberrant expression of miRNA has been linked to disease conditions including cancer (4). Mechanism of miRNA functions has been discussed in detail later in the review.
“Based on statistical data from 2004-2006, 1.40% of women born today will be diagnosed with ovarian cancer at some point during their lifetime (5).” Ovarian cancer is one of the factors responsible for the high mortality rate in women with gynecologic disorders in America to date (6). A common treatment for ovarian cancer is debulking surgery followed by platinum-based chemotherapy. Platinum- based chemotherapy typically comprises of carboplatin and paclitaxel and is usually given in six cycles (6). Unfortunately, while many patients do respond to the treatment initially, majority of them relapse due to chemo-resistance. The average survival of women with advanced ovarian cancer is 3-4 years and the relapse usually occurs within an average of 12- 18 months of initiating the treatment and continues during the course (6, 7). In an effort to understand, why patients relapse after a period, the patterns of gene expressions in ovarian cancer have been studied in clinical specimens and cell lines by employing cDNA microarray assays. Several genes are differentially expressed in ovarian cancer as deduced from the cDNA microarray assays that could possibly be the solution scientists are looking for in explaining chemo-resistance in certain ovarian cancers.
miRNAs are non-coding RNAs with average length of 22 nucleotides and have been implicated in posttranscriptional regulation of gene expression. Similar to messenger RNA (mRNA), miRNAs are transcribed by RNA polymerase II and are also spliced, and polyadenylated (8). However, in contrast to mRNAs, miRNAs are easily differentiated phenotypically due to its pre-miRNA containing stem-loop structure. This stem-loop structure is eventually cleaved by cytoplasmic RNase III dicer, which results in a miRNA duplex. The duplex strands are separated and one of the strands is degraded while the other one becomes a mature miRNA. Mature miRNAs are known to bind to complementary sequences in the 3' untranslated region (UTR) of the target mRNAs and usually causes silencing (i.e. suppression of translation) or cleavage of the mRNA (8).As modulators of protein expression, miRNAs have also been found to operate as both oncogenes and tumor suppressors (9, 10). Furthermore, miRNAs play roles in cell growth, differentiation, and programmed cell death because the levels of individual miRNAs are significantly different in varying cell types and different developmental stages (8). Moreover, these novel classes of regulators are now more commonly recognized as principal figures in essentially any development and progression of neoplasms (11).
The aim of this review paper is to highlight the miRNAs involved in causing a chemo-resistance by ovarian cancer cells against anticancer drugs. In addition, this will focus on the previously documented findings on the miRNAs expressed in various ovarian cancer cell lines. It will also list the miRNAs that play key roles in regulating other oncogenic pathways of the progression of ovarian cancer.
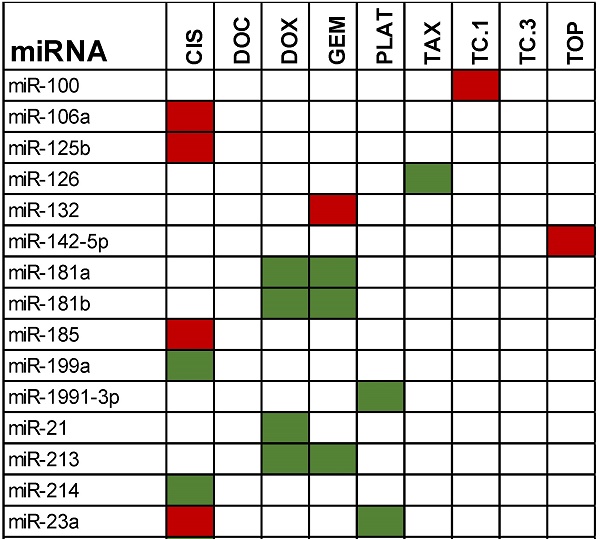
Table 1 gives an overview of the most recent miRNAs involved in producing chemo-resistance in patients with ovarian cancer. These miRNAs were mentioned by various investigators including, Sorrentino et al., 2008; Yang et al., 2008; Eitan et al, 2009; Boren et al, 2009(12); and Van Jaarsveld et al., 2010(13). Statistically significant miRNAs involved with inducing ovarian cancer are grouped based on the particular drug-resistance that was displayed during clinical and laboratory trials. The anti-cancer drugs used were cisplatin (cis), paclitaxel (tax), paclitaxel in the presence of cyclosporine as inhibitor of P-glycoprotein (TC1-TC3), doxorubicin (dox), docetaxel (doc), gemcitabine (gem), and topotecan (top). Sorrentino et al (2008), performed microarrays to analyze the miRNA profile in paclitaxel and cisplatin resistant cancerous cells and validated the results with qPCR and Northern blots (11). Their results showed that all the resistant cell lines expressed six particular miRNAs: Let-7e, miR-30c, miR-125b, miR-130a and miR-335. The expression of these 6 miRNAs were found to be deregulated in all the resistant cell lines, which suggested that there is a direct involvement of these miRNAs in the development of chemo-resistance.
Highly expressed miRNAs showing a resistance towards chemotherapeutic drugs. Green represents increased expression while red represents the miRNAs that were deregulated.
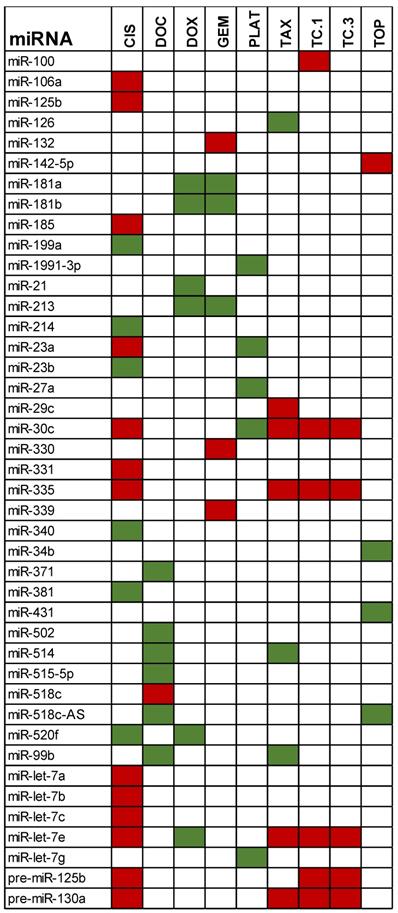
In another experiment(14), Nam et al. (2008) studied miRNA expression in clinical specimens and found that 23 miRNAs were deregulated with more than two fold change; they further analyzed the expression pattern by Northern blot analysis. Among the 23 miRNAs in their study, 6 (miR-125b, miR-100, let-7b, miR-23b, miR-27b and mir-21) showed similar expression pattern as found in the above study on the chemoresistant cell lines. It is significant that the expression of these miRNA was consistent in both cell lines and clinical specimens. Interestingly, the main finding in the cell line study was the link between miR-130a and the M-CSF gene. The M-CSF gene is a known resistance factor and enhancer of invasiveness and metastatic promoter for ovarian cancer(15). In silico studies using various computational methods showed M-CSF mRNA as a potential target for miR-130a (11, 16, 17). Furthermore, M-CSF protein is upregulated in all the chemo- resistant cell lines and this is in part caused by a marked reduction of miR-130a. A recent study on the relation of down regulation of miR-30a/c and cisplatin resistance shows a protein called Snail, which belongs to a family of zinc finger transcription factors, is the target of this micro RNA(18). The miR-30a/c-5p targets the 3'UTR of Snail mRNA. Recently, another work has also reported downregulation of miR-130a causing cisplatin resistance in ovarian cancer(19). This group reported miR-130a targets X-linked inhibitor of apoptosis (XIAP) indicating the diverse role of miR-130a in generating chemoresistance. In the study on clinical specimens (Nam et al, 2008), miR-199a was found to be down regulated and the authors considered this finding to be of importance in the development of chemoresistance and as a predictable marker for chemoresistant disease.
Yang et al. (2008) similarly found an miRNA, which also had an influence on an independent cell-signaling pathway that supplemented towards neoplasia. They have reported frequent deregulation of miR-214 along with a cluster of miRNA in ovarian cancers [5]. The PTEN/Akt pathway of cell signaling is mainly involved in the regulation of the cell cycle; however, if mutated or negatively influenced on, PTEN will be inhibited, therefore, allowing the Akt pathway to allow the cell cycle to uncontrollably proliferate (20). Akt inhibits apoptosis from occurring, which then leads to an increase in the amount of cells. This increase in cells leads to a growth of tumors/ neoplasia. The 3'-UTR of the PTEN is targeted by miR-214, which leads to down-regulation of PTEN protein and thus activation of the Akt cell-signaling pathway. This process then induces cell survival and cisplatin resistance. Furthermore, Yang et al. also reported several other miRNAs that are deregulated in ovarian cancer: miR-199a, miR-200a, and miR-100. Lastly, an anomalous expression of these miRNAs was detected in about 50% of the ovarian cancers, especially in the late- stages of tumor progression and in high- grade tumors. Lately, miR-100, miR-199a-3p, and miR-214 were found to be the three of the 8 microRNAs that are down regulated in malignant bladder tissue; miR-200a was found to be up regulated in the same (21). However, miR-218 has been reported to be the one that is overexpressed and marks the malignant bladder cancer sensitive to cisplatin (22).
A study performed by Boren et al. in 2009, lists selected miRNAs and their predicted mRNA targets in ovarian cancer cell lines (OVCA) and its implication in chemotherapy. Twenty-seven miRNAs were found to be associated with resistance against six chemotherapeutic agents, e.g., cisplatin, doxorubicin, topotecan, paclitaxel, docetaxel, and gemcitabine; 16 OVCA cell lines were analyzed for their sensitivity (12). Eighteen of the 27 miRNAs showed an increased expression and other nine showed a decreased expression with increasing resistance to individual drugs. The names of the miRNAs are incorporated into Table 1.
In a clinical perspective of determining miRNA's role in chemo-resistance, Eitan et al. (2009), performed this study on patients who underwent surgical treatment for ovarian cancer. All of the patients received platinum-based chemotherapy and were at various stages of the disease (stage 1-3). Their main finding of high expression of hsa-miR-27a was identified as having a very poor prognosis in a sub-group of patients (9). Patients whose disease kept progressing even during the first line platinum based chemotherapy and patients who had the disease in the past and had a relapse within 6 months of completing first line of therapy were termed platinum- resistant. Based on their experiments, has-miR-27a, 23a, 30c, let-7g, and miR-199a-3p showed higher expression in the patients who were termed platinum- resistant. Interestingly, hsa-miR-449b, when highly expressed, showed increased sensitivity to chemotherapy and improved survival rate. Recently, has-miR449b has been reported to target about 52 mRNAs, out of which one is the histone deacetylase 1 (HDAC1) (23). Previous study with the prostate cancer cells showed miR-449a regulation of HDAC1 causes cell cycle arrest and apoptosis through suppressing retinoblastoma (Rb) phosphorylation (24).
The role of p53 in regulating cell cycle and apoptosis is well studied. P53 protein regulates several of the miRNAs reported earlier e.g. miR-34b and miR-200. Activation of p53 influences mesenchymal-epithelial transition through these two miRNAs. In addition to regulating miRNA expression, p53 can also affect miRNA processing (25).
While the role of miRNAs in inducing chemo-resistance in ovarian cancer is a relatively new topic, much work has been done to specify the key players involved in this phenomenon. Whether the miRNAs are upregulated, deregulated, or even involved with influencing another pro-oncogenic pathway, the fact remains that some miRNAs cause chemo-resistance in ovarian cancer in-vitro and in-vivo. These miRNAs could serve as potential biomarkers for prognosis and chemotherapy resistance. Although further clinical studies are required to confirm these recent findings, these miRNAs present as potential candidates for therapeutic treatments for patients with ovarian cancer. Furthermore, this could be a solution to customize chemotherapy for individual cancer patient needs. This may help in selecting the most appropriate treatment for the patients who suffer from a high recurrence risk. Lastly, it could be used to counsel patients and allow doctors to plan for future treatments. While ovarian cancer is known to be the leading cause of gynecologic cancer deaths, recent evidence from translational studies gives us hope to battle this disease as the miRNA signatures discovered may be useful in categorizing, detecting, and predicting, as well as treating the different courses of ovarian cancer. In addition, miRNA in combination with anti-cancer drugs can be potentially more effective in combating these killer diseases.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-54
2. Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673-6
3. Hannon GJ. RNA interference. Nature. 2002;418:244-51
4. Di Leva G, Croce CM. The Role of microRNAs in the Tumorigenesis of Ovarian Cancer. Front Oncol. 2013;3:153
5. Adams SA, Hebert JR, Bolick-Aldrich S, Daguise VG, Mosley CM, Modayil MV. et al. Breast cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J S C Med Assoc. 2006;102:231-9
6. Friedlander M, Butow P, Stockler M, Gainford C, Martyn J, Oza A. et al. Symptom control in patients with recurrent ovarian cancer: measuring the benefit of palliative chemotherapy in women with platinum refractory/resistant ovarian cancer. Int J Gynecol Cancer. 2009;19(Suppl 2):S44-8
7. Blackledge G, Lawton F, Redman C, Kelly K. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989;59:650-3
8. Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J. et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425-33
9. Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M. et al. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253-9
10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-33
11. Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478-86
12. Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Chan G. et al. MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol Oncol. 2009;113:249-55
13. van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA. MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol. 2010;42:1282-90
14. Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH. et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690-5
15. Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429-40
16. Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39-48
17. Foti E, Ferrandina G, Martucci R, Romanini ME, Benedetti Panici P, Testa U. et al. IL-6, M-CSF and IAP cytokines in ovarian cancer: simultaneous assessment of serum levels. Oncology. 1999;57:211-5
18. Han X, Zhen S, Ye Z, Lu J, Wang L, Li P. et al. A Feedback Loop Between miR-30a/c-5p and DNMT1 Mediates Cisplatin Resistance in Ovarian Cancer Cells. Cell Physiol Biochem. 2017;41:973-86
19. Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai). 2013;45:995-1001
20. Xu N, Lao Y, Zhang Y, Gillespie DA. Akt: a double-edged sword in cell proliferation and genome stability. J Oncol. 2012;2012:951724
21. Ratert N, Meyer HA, Jung M, Lioudmer P, Mollenkopf HJ, Wagner I. et al. miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J Mol Diagn. 2013;15:695-705
22. Li P, Yang X, Cheng Y, Zhang X, Yang C, Deng X. et al. MicroRNA-218 Increases the Sensitivity of Bladder Cancer to Cisplatin by Targeting Glut1. Cell Physiol Biochem. 2017;41:921-32
23. Buggele WA, Krause KE, Horvath CM. Small RNA profiling of influenza A virus-infected cells identifies miR-449b as a regulator of histone deacetylase 1 and interferon beta. PLoS One. 2013;8:e76560
24. Noonan EJ, Place RF, Basak S, Pookot D, Li LC. miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells. Oncotarget. 2010;1:349-58
25. Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613-26
Author contact
![]() Corresponding author: Tel. 281-283-3756; E-mail: rashidedu
Corresponding author: Tel. 281-283-3756; E-mail: rashidedu