J Biomed 2018; 3:1-7. doi:10.7150/jbm.20966 This volume Cite
Review
Drug development of Lung Diseases by way of Drug Repurposing; What a Pulmonary Physician can do in the Laboratory
1. Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece;
2. Sana Clinic Group Franken, Department of Cardiology / Pulmonology / Intensive Care / Nephrology, ''Hof'' Clinics, University of Erlangen, Hof, Germany;
3. Department of Pharmacology & Clinical Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece;
4. Department of Respiratory and Critical Care Medicine, Changhai Hospital/First Affiliated Hospital of the Secondary Military Medical University, Shanghai, China;
5. Johns Hopkins University, Pulmonary and Critical Care, Baltimore, Maryland, United States;
6. Surgery Department, “Interbalkan European Medical Center”, Thessaloniki, Greece;
7. Thoracic Surgery Department, “Theageneio” Anticancer Center, Thessaloniki, Greece;
8. Interventional Pulmonary Department, “Ruhrland Klinic”, University of Duisburg-Essen, Essen, Germany;
9. Research Laboratory and International Collaboration, Bon Secours Cancer Institute, VA, USA.
Received 2017-5-10; Accepted 2017-8-4; Published 2017-11-1
Abstract
Lung diseases are very common among several patients such as; asthma, chronic obstructive pulmonary disease, lung cancer, cystic fibrosis and pulmonary fibrosis. In many of these diseases we already have inhalation therapies, however; for others we have only systematic administration. There are also diseases of the lung that cause pulmonary hypertension and architectural damage to the lung such as; emphysema and bronchiectasis. Several of the drugs currently administered intravenously are being investigated whether they could be transformed and delivered as aerosol therapy. We will present the methodology that can be used and has been used for several drugs.
Keywords: aerosol, devices, inhalation, milling, mastersizer, droplets, particles.
Introduction
Currently there are several drugs that are being administered by inhalation either as aerosol or powder for lung diseases. It is very well known that for asthma and chronic obstructive disease (COPD) we use nebulisers, metered dose inhalers (MDIs), and dry powder inhalers (DPIs) for the delivery of inhalational bronchodilators and corticosteroids [1]. Moreover; there are inhaled antibiotics and inhaled drugs for pulmonary hypertension already on the market [2, 3]. It is known that there are several factors affecting the distribution and absorption of an inhalational compound. Also, the administration is affected by several factors. The distribution and deposition is affected by the stage of the underlying disease and whether there is exacerbation when the drug is administered, the mass median aerodynamic diameter (mmad) of the particles (should be <5μm), local transporters and gene expression on the bronchial tree, humidity of the airways and solubility. These factors will be thoroughly explained in the following sections [4]. In the current work we will present methods that can be easily used by clinical doctors in the laboratory in order to repurpose a drug from one form to another based on previous published work from our institutions.
Transformation methodology
In several experiments that transformed a drug from powder or pill to aerosol, usually the procedure of milling was used with a planetary ball mill (Frisch, Pulverisette-5) equipped with Agate bowls (500 ml) and 8 balls (20 mm, 20 g) with a high rotational speed of approximately 200 rpm. Drugs such tyrosine kinase inhibitors (pills) and antibiotics (pills/powder) used this method to transform the pills into powder or decrease the size of the powder in order to have mmad <5μm (Figure 1). In order to measure the mmad we can use a cascade impactor or a mastersizer system. A mastersizer system is easier to use in a laboratory, while the cascade impact is usually used in the industry (Figure 2). The size distribution of the droplets and their mean diameter (d32) can be calculated using a Malvern Mastersizer 2000 apparatus (Malvern Instruments Ltd, Malvern, Worcestershire, UK) equipped with a Scirocco module (Malvern Instruments Ltd, Malvern, Worcestershire, UK). The device can be modified as for the user to be able to spray the generated droplets/powder directly perpendicular to the laser beam (i.e. [5-9]). A refractive index of 1.33 is used for the sprayed droplets. The measurements are performed under ambient temperature. For these measurements to be accurate especially when aerosol droplets are being measured we have to take into serious consideration the solubility of the compound, if the liquid is extremely colloidal then the measurement has to be performed several times and the apparatus has to cleaned thoroughly between the measurements, and for each measurement. Moreover; pharmaceutical companies often use freeze-drying to increase the shelf life of the products, such as live virus vaccines, biologics and other injectables. By removing the water from the material and sealing the material in a glass vial, the material can be easily stored, shipped, and later reconstituted to its original form for injection. Another example from the pharmaceutical industry is the use of freeze drying to produce tablets or wafers, the advantage of which is less excipient as well as a rapidly absorbed and easily administered dosage form. Freeze-drying is a dehydration process typically used to preserve a perishable material or make the material more convenient for transport. Freeze-drying works by freezing the material and then reducing the surrounding pressure to allow the frozen water in the material to sublime directly from the solid phase to the gas phase.
Milling equipment

Mastersizer 2000

Parameters affecting the drug development and experimentation
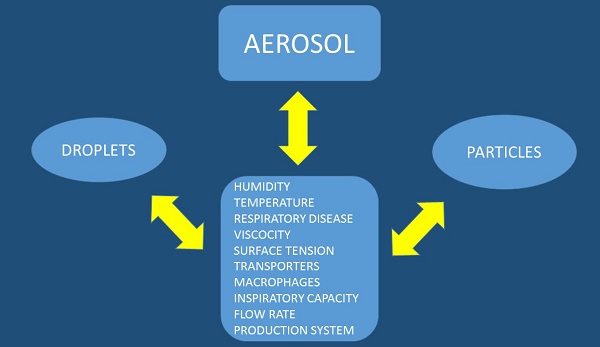
Aerosol Administration
There are several factors affecting the production and deposition of aerosol droplets or particles (Figure 3). We should try and divide these two methods of drug administration separately. Aerosol droplets need a system which will make a solution into aerosol in this case we can use a nebulization system which could be an electrical powered device or mechanical powered device with a springer such as with the case of Spiriva Respimate®. Regarding the production system for aerosol droplets the following parameters have to be considered: a) design of the residual cup, b) nebulizer oxygen or pressure flow rate, c) taping the residual cup during nebulization, d) residual cup loading, e) chemical structure of the drug, f) viscosity, and g) surface tension. The Regarding the aerosol particles the following parameters should be considered upon design of the experiment: a) inspiratory capability of the patient, b) the particle should have both x and y axes as close as possible, otherwise if one is longer then it is more irritating for the respiratory tract and cough is more often, c) drug concentration, d) drug structure, e) saving the drug in a room storage under conditions that do not induce grinding. In any case for both methods the following should be considered which are connected with the site of the drug deposition within the respiratory airways: a) humidity (>99%), b) airway temperature (37°C), c) surface tension, d) droplet or particle size less than <5μm (otherwise the drug will remain in the upper respiratory system) and e) underlying respiratory disease or exacerbation of the disease (e.g. asthma, chronic obstruction pulmonary disease-COPD, interstitial pulmonary fibrosis-IPF, lung cancer, cystic fibrosis, atelectasis and thick mucus). Regarding humidity; it has been observed that a particle or droplet size could increase up to 50% while travelling through the airways from the upper to the lower respiratory tract [10]. Moreover; increased salt in a drug increases water concentration in a particle or droplet up to 25%. Local factors of the airways such as the macrophages, transporters or local gene expression play a role in the absorption of the drug and these are parameters that can be modified. The patient target group should be considered as some devices need synchronization upon device activation and patient inspiration [4, 10-23]. Breathing patterns also modify the deposition of aerosol within the airways, and therefore correct usage of inhalable devices is necessary. Moreover; deep inspiratory efforts are necessary for efficient aerosol deposition. Underlying disease affects the inspiratory capability of a patient and therefore the appropriate administration device must be chosen for each patient, or even might change upon disease exacerbation [20].
Lung cancer treatment
In the past ten years more than ten non-specific anticancer drugs have been administered as aerosol either in the setting of a clinical trial (Phase I/II) or an animal laboratory [24]. Moreover; novel tyrosine kinase inhibitors (TKIs) have been also transformed to aerosol formulations with the concept to be administered in future studies [25]. The following drugs have been investigated as aerosol administration or dry powder; gemcitabine, carboplatin, cisplatin, bevacisumab, paclitaxel and docetaxel [24]. Regarding TKIs there are no clinical data as local treatment for now, however; regarding non-specific anticancer agents there are clinical data in favor of this local treatment modality. However; the safety issue still remains since adverse effects such as non-specific inflammation of the airways or pulmonary edema was observed at least for gemcitabine and paclitaxel [24]. We should take under consideration that the formulations administered were not designed for the airways therefore possibly novel compounds should be considered [26, 27]. Moreover; all trials were performed either in patients with advanced stage disease or as a short term animal experiments, therefore the safety matter was not properly assessed since an extended time of observation at least more than 6 months is necessary. In the studies by Zarogoulidis P. et al. [15, 28] it was observed that the residual cup design along with the jet-nebuliser could increase the small droplet production. Also, the clinical translation of the aerosol production combination was performed in NSCLC patients. Until now there is only one study that was performed for early stage operable lung cancer with the concept to check the infiltration of the mediastinal lymph nodes from aerosol cisplatin. In this study the aerosol cisplatin was administered before curative surgery [29].
Pulmonary Hypertension
Pulmonary hypertension occurs either idiopathically or is caused by several underlying conditions. In some situations it is life-threatening and therefore early diagnosis and treatment is necessary. Currently treatment is administered by several methods such as; intravenously with a pump, oral, subcutanesously and as aerosol. Iloprost and treprostinil were two of the first drugs to be administered as aerosol. Nitric oxide, sildenafil and epoprostenol more recently are also used as aerosol [30, 31]. Tyrosine kinase inhibitors such imatinib, erlotinib, gefitinib have been also investigated whether they could be administered as aerosol. However; in the study by Pitsiou G. et al. [25] it was observed that gefitinib could not be transformed at least with the presented methodology as aerosol. Bosetan was also transformed in order to be delivered as aerosol [31]. In the study by Huang H. et al. [31] with the drugs bosentan, pirfenidone, treprostinil and sildenafil after milling and several experiments it was observed that the role of the residual cup design and jet-nebulizer choice were the most important factors for small aerosol droplets. Moreover; a novel rho-kinase inhibitor called fasudil was administered as aerosol in mice and the current study presented favorable data that the administered compound could be a future treatment [32].
Antibiotics
Inhaled antibiotics are on the market for several years now. Several antibiotic compounds were introduced in the market along with a specific device that was appropriate to deliver more efficiently the powder or aerosol by inhalation [2]. The following antibiotics are already on the market or have been investigated for aerosol administration; Tobramycin, Aztreonam, Gentamicin, Amikacin, Fosfomycin, Clolistin, Amphotericin, Isoniasid, Rifampicin, Capreomycin, Levofloxacin, Ciprofloxacin, Doxycycline, Clindamycin and Telithromycin. Several of the antibiotics were administered in their raw form or as a novel compound in order to enhance the absorption [2]. The different methods of the aerosol administration can be found on Table 1 [33-47]. The drugs have been administered for pulmonary infections in several conditions such as; pneumonia, cystic fibrosis and bronchiectasis. The major adverse effects were cough, dysgeusia, dysphonia, bronchospasm, pharyngitis, pneumothorax, tinnitus wheezing and hoarseness of voice. In the experiments by Zarogoulidis P. et al. [9, 48] with ampicillin-sulbactam, meropenem, ceftazidime, cefepime, piperacillin-tazobactam, linezolid, vancomycin and daptomycin it was observed that again the residual cup design along with specific jet-nebulizer compressor parameters were the most important factors affecting the small droplet production.
Discussion
We still need a universal respiratory model in order to quickly and efficiently investigate the safety and efficiency of novel molecules. In Figure 4, a pig lung is used right after it was euthanized, we used a pump with several connecting systems in the large vessels in order to simulate a living animal. The lung is inside a plastic box with negative pressure simulating the respiration movement, and through the trachea aerosol compounds are administered. We have only four hours until edema occurs in the alveoli. Taking alveolar lavage after aerosol administration of tissue samples we can evaluate the short term toxicity of each drug administered. This is currently our methodology until we evaluate and agree on other respiratory models.
In the next five years we expect new drugs for pulmonary hypertension and pulmonary fibrosis to be designed and administered in clinical trials in the form of a sustain release system with long term absorption. Regarding chronic obstructive disease, there is currently an effort to produce novel molecules combining drugs such as; bronchodilators plus corticosteroids, plus ipratropium and simultaneously keep a small droplet/particle size in order to efficiently penetrate deep into the lung parenchyma. Table 1.
Conclusion
Novel molecules that can combine several are necessary as in the case of COPD where a triple action treatment is necessary to deliver simultaneously a bronchodilator, corticosteroid and ipratropium bromide. An important factor affecting the inhalational treatment is the toxicity of drugs to the lung parenchyma, therefore preclinical studies are necessary in cell lines and possibly in animals. The underlying disease and disease stage much be taken into account before administering a drug or before choosing a drug for administration. In order to administer a drug as aerosol or powder we need to have a device which will enable the administration and possibly enhance the drug delivery.
Methods and Models of aerosol deposition evaluation and enhancement
| Durand M, Pourchez J, Aubert G, Le Guellec S, Navarro L, Forest V, Rusch P, and Cottier M. | Deposition evaluation model with classic nebulizer or 100Hz acoustic airflow |
| McCormack P, McNamara PS, and Southern KW. | Two different breathing modes were evaluated |
| Willis LD and Berlinski A. | Survey on aerosol administration in tracheostomized children by pediatric pulmonologists |
| Vecellio L, Abdelrahim ME, Montharu J, Galle J, Diot P, and Dubus JC. | Disposable versus reusable jet nebulizers |
| Stegen K, Neujens A, Crombez G, Hermans D, Van de Woestijne KP, and Van den Bergh O. | Negative affect of CO2 addition in nebulization |
| Caille V, Ehrmann S, Boissinot E, Perrotin D, Diot P, and Dequin PF. | Nasal additional oxygen delivery during air-driven jet nebulization increases FiO2 |
| Britland S, Finter W, Chrystyn H, Eagland D, and Abdelrahim ME. | Different aerosol formulations interact differently with the solutions and tissue in the respiratory system. |
| Coates AL, Green M, Leung K, Chan J, Ribeiro N, Ratjen F, and Charron M. | Superiority of the investigational eFlow® by producing the same amount of aerosol in half time in comparison to PARI LC PLUS® |
| Pitance L, Reychler G, Leal T, Reychler H, Liistro G, Montharu J, Lab T, Diot P, and Vecellio L. | Sidestream® jet nebulizer with and without corrugated piece of tubing |
| Wee WB, Leung K, and Coates AL. | A proposed aerosol evaluation model i) mathematical model derivation, ii) in vitro testing and iii) in vivo testing. |
| Tiemersma S, Minocchieri S, Lingen RA, Nelle M, and Devadason SG. | eFlow® nebulizer system more efficient in comparison to Intersurgical® Cirrus jet® nebulizer and pressured meter dose inhaler with an Aerochamber® for drug delivery to preterm infants |
| Pitance L, Vecellio L, Leal T, Reychler G, Reychler H, and Liistro G. | Sidestream® jet nebulizer with and without corrugated piece of tubing in six healthy spontaneous breathing volunteers |
| Rao N, Kadrichu N, and Ament B. | Refrigerating the impactor down to 5oC prior to aerosol measurement produced by vibrating mesh vebulizers |
| McCormack P, Southern KW, and McNamara PS. | Automatic data recording of patient adherence to aerosol administration |
| Skaria S and Smaldone GC. | Omron NE U22 was evaluated in comparison to Pari LC Plus® and Sidestream® |
| Fadl A, Wang J, and Zhang Z. | Metered dose inhaler mouthpieces were modified in order reduce the inertial impaction in order to reduce aerosol deposition to the oral airway |
Blue arrow indicates the pump, red arrow indicates the trachea entry, black arrow indicates a large vessel connection

Cisplatin powder under confocal microscope
Competing Interests
The authors have declared that no competing interest exists.
References
1. Braithwaite I, Williams M, Power S, Pilcher J, Weatherall M, Baines A. et al. Randomised, double-blind, placebo-controlled, cross-over single dose study of the bronchodilator duration of action of combination fluticasone furoate/vilanterol inhaler in adult asthma. Respiratory medicine. 2016;119:115-21 doi:10.1016/j.rmed.2016.09.006
2. Zarogoulidis P, Kioumis I, Porpodis K, Spyratos D, Tsakiridis K, Huang H. et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug design, development and therapy. 2013;7:1115-34 doi:10.2147/DDDT.S51303
3. Gabrielli L, Ocaranza MP, Sitges M, Kanacri A, Saavedra R, Sepulveda P. et al. Acute effect of iloprost inhalation on right atrial function and ventricular dyssynchrony in patients with pulmonary artery hypertension. Echocardiography. 2016 doi:10.1111/echo.13401
4. Zarogoulidis P, Darwiche K, Yarmus L, Spyratos D, Secen N, Hohenforst-Schmidt W. et al. Defense mechanisms of the respiratory system and aerosol production systems. Medicinal chemistry. 2014;10:123-36
5. Zarogoulidis P, Darwiche K, Huang H, Spyratos D, Yarmus L, Li Q. et al. Time recall; future concept of chronomodulating chemotherapy for cancer. Curr Pharm Biotechnol. 2013;14:632-42 doi:CPB-EPUB-56654 [pii]
6. Zaric B, Stojsic V, Tepavac A, Sarcev T, Zarogoulidis P, Darwiche K. et al. Adjuvant chemotherapy and radiotherapy in the treatment of non-small cell lung cancer (NSCLC). J Thorac Dis. 2013;5:S371-S7 doi:10.3978/j.issn.2072-1439.2013.05.16 jtd-05-S4-S371 [pii]
7. Boukovinas I, Tsakiridis K, Zarogoulidis P, Machairiotis N, Katsikogiannis N, Kougioumtzi I. et al. Neo-adjuvant chemotherapy in early stage non-small cell lung cancer. J Thorac Dis. 2013;5:S446-S8 doi:10.3978/j.issn.2072-1439.2013.07.36 jtd-05-S4-S446 [pii]
8. Zarogoulidis P, Darwiche K, Tsakiridis K, Teschler H, Yarmus L, Zarogoulidis K. et al. Learning from the Cardiologists and Developing Eluting Stents Targeting the Mtor Pathway for Pulmonary Application; A Future Concept for Tracheal Stenosis. Journal of molecular and genetic medicine: an international journal of biomedical research. 2013;7:65. doi:10.4172/1747-0862.1000065
9. Zarogoulidis P, Kioumis I, Ritzoulis C, Petridis D, Darwiche K, Porpodis K. et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam. International journal of pharmaceutics. 2013;455:182-8 doi:10.1016/j.ijpharm.2013.07.040
10. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. British journal of clinical pharmacology. 2003;56:588-99
11. Clay MM, Pavia D, Newman SP, Clarke SW. Factors influencing the size distribution of aerosols from jet nebulisers. Thorax. 1983;38:755-9
12. Smith EC, Denyer J, Kendrick AH. Comparison of twenty three nebulizer/compressor combinations for domiciliary use. The European respiratory journal. 1995;8:1214-21
13. Douglas JG, Leslie MJ, Crompton GK, Grant IW. Is the flow rate used to drive a jet nebuliser clinically important? British medical journal. 1985;290:29
14. Newman SP, Pellow PG, Clay MM, Clarke SW. Evaluation of jet nebulisers for use with gentamicin solution. Thorax. 1985;40:671-6
15. Zarogoulidis P, Petridis D, Ritzoulis C, Darwiche K, Spyratos D, Huang H. et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: back to drawing the residual cup. International journal of pharmaceutics. 2013;453:480-7 doi:10.1016/j.ijpharm.2013.06.011
16. Kendrick AH, Smith EC, Denyer J. Nebulizers-fill volume, residual volume and matching of nebulizer to compressor. Respiratory medicine. 1995;89:157-9
17. Kendrick AH, Smith EC, Wilson RS. Selecting and using nebuliser equipment. Thorax. 1997;52(Suppl 2):S92-101
18. Kwok PC, Trietsch SJ, Kumon M, Chan HK. Electrostatic charge characteristics of jet nebulized aerosols. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:149-59 doi:10.1089/jamp.2009.0795
19. Davis MS, Foster WM. Airflow and device effects on aerosol delivery for large animals. Journal of veterinary pharmacology and therapeutics. 2001;24:57-60
20. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. British journal of clinical pharmacology. 2003;56:600-12
21. Zarogoulidis P, Papanas N, Kouliatsis G, Spyratos D, Zarogoulidis K, Maltezos E. Inhaled insulin: too soon to be forgotten? Journal of aerosol medicine and pulmonary drug delivery. 2011;24:213-23 doi:10.1089/jamp.2011.0876
22. Suresh Babu K, Kastelik J, Morjaria JB. Role of long term antibiotics in chronic respiratory diseases. Respiratory medicine. 2013;107:800-15 doi:10.1016/j.rmed.2013.02.009
23. Togami K, Chono S, Morimoto K. Aerosol-based efficient delivery of clarithromycin, a macrolide antimicrobial agent, to lung epithelial lining fluid and alveolar macrophages for treatment of respiratory infections. Journal of aerosol medicine and pulmonary drug delivery. 2012;25:110-5 doi:10.1089/jamp.2011.0894
24. Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP. et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. International journal of nanomedicine. 2012;7:1551-72 doi:10.2147/IJN.S29997
25. Pitsiou G, Zarogoulidis P, Petridis D, Kioumis I, Lampaki S, Organtzis J. et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug design, development and therapy. 2014;8:1753-63 doi:10.2147/DDDT.S70277
26. Zarogoulidis P, Giraleli C, Karamanos NK. Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered. Therapeutic delivery. 2012;3:1021-3
27. Darwiche K, Zarogoulidis P, Karamanos NK, Domvri K, Chatzaki E, Constantinidis TC. et al. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology. Future oncology. 2013;9:505-25 doi:10.2217/fon.12.205
28. Zarogoulidis P, Eleftheriadou E, Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T. et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investigational new drugs. 2012;30:1628-40 doi:10.1007/s10637-011-9714-5
29. Zarogoulidis P, Darwiche K, Krauss L, Huang H, Zachariadis GA, Katsavou A. et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future oncology. 2013;9:1307-13 doi:10.2217/fon.13.111
30. Hill NS, Preston IR, Roberts KE. Inhaled Therapies for Pulmonary Hypertension. Respiratory care. 2015;60:794-802 discussion -5. doi:10.4187/respcare.03927
31. Huang H, Zarogoulidis P, Lampaki S, Organtzis J, Petridis D, Porpodis K. et al. Experimentation with aerosol bonsetan, pirfenidone, treprostinil and sidenafil. Journal of thoracic disease. 2014;6:1411-9 doi:10.3978/j.issn.2072-1439.2014.08.38
32. Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M. et al. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. Journal of controlled release: official journal of the Controlled Release Society. 2013;167:189-99 doi:10.1016/j.jconrel.2013.01.011
33. Durand M, Pourchez J, Aubert G, Le Guellec S, Navarro L, Forest V. et al. Impact of acoustic airflow nebulization on intrasinus drug deposition of a human plastinated nasal cast: new insights into the mechanisms involved. International journal of pharmaceutics. 2011;421:63-71 doi:10.1016/j.ijpharm.2011.09.023
34. Willis LD, Berlinski A. Survey of aerosol delivery techniques to spontaneously breathing tracheostomized children. Respiratory care. 2012;57:1234-41 doi:10.4187/respcare.01518
35. McCormack P, McNamara PS, Southern KW. A randomised controlled trial of breathing modes for adaptive aerosol delivery in children with cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2011;10:343-9 doi:10.1016/j.jcf.2011.04.006
36. Wee WB, Leung K, Coates AL. Modeling breath-enhanced jet nebulizers to estimate pulmonary drug deposition. Journal of aerosol medicine and pulmonary drug delivery. 2013;26:387-96 doi:10.1089/jamp.2012.0984
37. Stegen K, Neujens A, Crombez G, Hermans D, Van de Woestijne KP, Van den Bergh O. Negative affect, respiratory reactivity, and somatic complaints in a CO2 enriched air inhalation paradigm. Biological psychology. 1998;49:109-22
38. Caille V, Ehrmann S, Boissinot E, Perrotin D, Diot P, Dequin PF. Influence of jet nebulization and oxygen delivery on the fraction of inspired oxygen: an experimental model. Journal of aerosol medicine and pulmonary drug delivery. 2009;22:255-61 doi:10.1089/jamp.2008.0718
39. Vecellio L, Abdelrahim ME, Montharu J, Galle J, Diot P, Dubus JC. Disposable versus reusable jet nebulizers for cystic fibrosis treatment with tobramycin. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2011;10:86-92 doi:10.1016/j.jcf.2010.10.004
40. Coates AL, Green M, Leung K, Chan J, Ribeiro N, Ratjen F. et al. A comparison of amount and speed of deposition between the PARI LC STAR(R) jet nebulizer and an investigational eFlow(R) nebulizer. Journal of aerosol medicine and pulmonary drug delivery. 2011;24:157-63 doi:10.1089/jamp.2010.0861
41. Tiemersma S, Minocchieri S, van Lingen RA, Nelle M, Devadason SG. Vibrating membrane devices deliver aerosols more efficient than standard devices: a study in a neonatal upper airway model. Journal of aerosol medicine and pulmonary drug delivery. 2013;26:280-6 doi:10.1089/jamp.2012.0993
42. Pitance L, Reychler G, Leal T, Reychler H, Liistro G, Montharu J. et al. Aerosol delivery to the lung is more efficient using an extension with a standard jet nebulizer than an open-vent jet nebulizer. Journal of aerosol medicine and pulmonary drug delivery. 2013;26:208-14 doi:10.1089/jamp.2012.0994
43. Pitance L, Vecellio L, Leal T, Reychler G, Reychler H, Liistro G. Delivery efficacy of a vibrating mesh nebulizer and a jet nebulizer under different configurations. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:389-96 doi:10.1089/jamp.2010.0816
44. McCormack P, Southern KW, McNamara PS. New nebulizer technology to monitor adherence and nebulizer performance in cystic fibrosis. Journal of aerosol medicine and pulmonary drug delivery. 2012;25:307-9 doi:10.1089/jamp.2011.0934
45. Rao N, Kadrichu N, Ament B. Application of a droplet evaporation model to aerodynamic size measurement of drug aerosols generated by a vibrating mesh nebulizer. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:295-302 doi:10.1089/jamp.2009.0805
46. Skaria S, Smaldone GC. Omron NE U22: Comparison between vibrating mesh and jet nebulizer. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:173-80 doi:10.1089/jamp.2010.0817
47. Fadl A, Wang J, Zhang Z. Metered-dose inhaler efficiency enhancement: a case study and novel design. Inhalation toxicology. 2010;22:601-9 doi:10.3109/08958371003599029
48. Zarogoulidis P, Kioumis I, Lampaki S, Organtzis J, Porpodis K, Spyratos D. et al. Optimization of nebulized delivery of linezolid, daptomycin, and vancomycin aerosol. Drug design, development and therapy. 2014;8:1065-72 doi:10.2147/DDDT.S66576
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom
Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom